Abstract
Background and Objective :Maintenance therapy (MT) post-induction or post autologous stem cell transplant (ASCT) in newly diagnosed Multiple Myeloma (NDMM) patients represents a vital strategy to delay the disease progression and relapse. Lenalidomide (LEN) was the first FDA-approved oral drug in MT after ASCT in Multiple Myeloma(MM). Bortezomib (BTZ) is a first-in-class proteasome inhibitor, widely used as induction therapy for NDMM. Unlike LEN, there is less published data on the role of BTZ in MT. The availability of generic drugs and subcutaneous route of administration has made BTZ a preferred drug for MT in low/middle income countries (LMICs) like India. Our objective was to analyze the regional real world data on outcomes between BTZ based Vs LEN based MT in NDMM.
Method : Following institutional review board approval, the electronic medical records of consecutive NDMM patients diagnosed from January 2013 to December 2020 at two centers in India were screened retrospectively. All patients who received induction+/- ASCT followed by either LEN or BTZ-based MT were included in the analysis. Those not started on MT or lost to follow-up before MT were excluded. The data included demographics and clinical characteristics like International Staging System (ISS), induction regimen, ASCT, MT, adverse events, relapse and response up to the last follow-up date. Response was assessed as per the International Myeloma Working Group (IMWG) Criteria. BTZ MT was defined as (1.3 mg/m2) once every two weeks, administered subcutaneously alone or in combination with other drugs, whereas LEN MT was administered as oral tablets (10 mg/ day increased up to 15mg/day x 21/28 days based on drug tolerance). BTZ in combination with LEN was considered under BTZ MT during the analysis. Progression free survival (PFS) was calculated from the date of initiation of induction to relapse or death whichever earlier and overall survival (OS) from the date of initiation of induction till death.Time -to -event analyses were performed using the Kaplan -Meier (KM ) method to assess the PFS and OS of both groups who were started on BTZ and LEN based MT and those who completed one year of MT. The log -rank test was used to assess the differences between the groups. P value <0.05 was considered to be significant. All statistical analyzes were performed using IBM SPSS Version 26 Software.
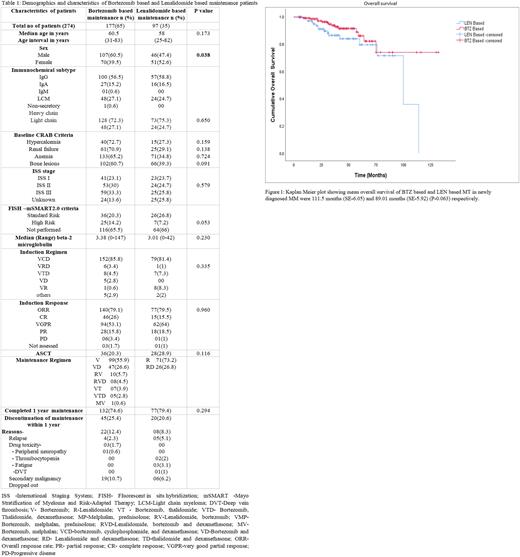
Results: Electronic medical records of 550 consecutive patients diagnosed with MM during the study period were screened and 431 patients were identified as NDMM. Of these, 419 patients received induction, 71 underwent ASCT and 274 received MT. Among these 274 patients, 177(65%) received BTZ based, while 97(35%) received LEN-based MT. The median age was 60.5 ( range 31-83)years in BTZ based MT and 58 (range 25-82)years in LEN based MT. Demographics, baseline characteristics, induction, ASCT, MT regimen, and the response of these patients are detailed in Table 1. Both groups were comparable across most variables. Most common Induction regimen received by patients of both groups was Bortezomib Cyclophosphamide and Dexamethasone (VCD)( 85.8% in BTZ based MT vs. 81.4% in LEN based MT ;p-0.335);Of these 20.3 % and 28.9% (p 0.116) underwent ASCT respectively. Of the patients who received MT,132 patients (74.5%) in BTZ based and 77 (79.3%) in LEN based group completed one year of maintenance. The reasons for discontinuation in the first year were relapse, toxicity and loss to follow up (Ref Table 1 ).The mean PFS estimate for patients who were started on BTZ based MT was 61.13 months (SE-5.18) and 45.16 months (SE-3.92) (P-0.203) for LEN based MT.The corresponding mean KM OS estimated for BTZ and LEN based MT were 111.5 months (SE-6.05) and 89.01 months (SE-5.92) respectively (P-0.063)(Ref Fig 1)
Conclusion: In this real world analysis BTZ based MT appears feasible, safe and effective for NDMM. Our study was limited by its retrospective nature. Risk stratification by Fluorescent in situ Hybridization was not widely available. Patients who underwent ASCT were limited in number even in those who were eligible , underlining the probable difficulty to access the expenses of this treatment in a LMIC. Comparatively MT appeared much more accessible, likely due to the availability of generic BTZ and LEN in India. Similar outcomes for PFS and OS in both MT groups points towards the need for prospective trials with BTZ and LEN based MT regimens for different risk sub groups in the region.
Disclosures
Sidharthan:Astra Zeneca: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; jansen: Speakers Bureau; Emcure: Consultancy; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Bortezomib and its use in maintenance therapy in Newly Diagnosed Multiple Myeloma
Author notes
Asterisk with author names denotes non-ASH members.